A Comprehensive COMSOL Modeling for the Solar-Driven CO2 Electroreduction to CO
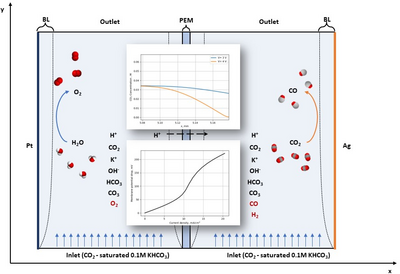
Climate change is a short-term problem which can no longer be ignored and affects everything, from global food security to geopolitics and economics: extreme weather phenomena, falling crops yields, decrease of water availability, sea level rise, extinction of several animal species, are some of the many catastrophic consequences related to global warming. Carbon dioxide (CO2) is the primary greenhouse gas which contributes to climate change, due to its high residence time and radiative forcing. CO2 emission is primarily associated with the combustion of fossil fuels (which fulfills the 80% of the worldwide energy demand), heat generation and electricity. Growing demand in energy market speeds up the global energy consumption, leading to an increasing trend of CO2 concentration in air. In this framework, electrochemistry is the most promising process for decarbonization, since it allows the conversion of CO2 into added-value products in an up-scalable and sustainable technology. In this work, COMSOL Multiphysics 6.1 software has been exploited to produce a comprehensive model for the electrochemical cell and validated through experimental results with good agreement. A 2D model is developed consisting of cathode and anode domains separated by an ion exchange membrane: Tertiary Current Distribution (TCD) has been employed for the cathodic and anodic compartments for the electrochemical and equilibrium reactions inside the electrolyte, and it has been coupled with a Secondary Current Distribution (SCD) physics for the ion exchange membrane domain. The catalysts for Oxygen Evolution Reaction (OER) and CO2 reduction reaction (CO2RR) (Platinum foil and Silver nanoparticles, respectively) are modeled by infinitely thin films at the boundaries of the domain, by feeding the Butler-Volmer equations with their electrochemical parameters in the TCD physics. The model successfully replicates the experimental data for the conversion of CO2 into Carbon Monoxide (CO) and can predict the performance of the device by varying different parameters (e.g., voltage applied, inflow electrolyte velocity, dimension of the cell) [1]. Moreover, chemical conditions at the electrode surfaces can be inspected, such as variation of chemical species concentrations and pH in the diffusive boundary layers. In addition, the integration with photovoltaics as electrical source is implemented as well: the model simulates the electrochemical cell integrated with a Dye-Sensitized Solar Cells [2], such that the performance of the device as a function of the solar irradiance can be predicted. Modeling the electrochemical setup is a powerful tool not only to predict and optimize the operational conditions of the device, but also to follow the scaling-up process of the technology and its integration into more complex systems.