La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
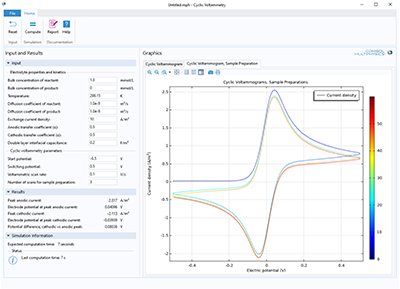
The purpose of the app is to demonstrate and simulate the use of cyclic voltammetry. You can vary the bulk concentration of both species, transport properties, kinetic parameters, as well as the cycling voltage window and scan rate. Cyclic voltammetry is a common analytical technique ... En savoir plus
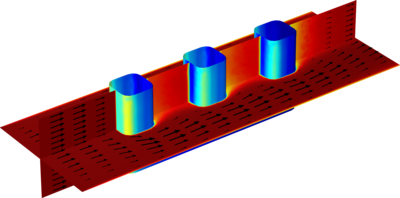
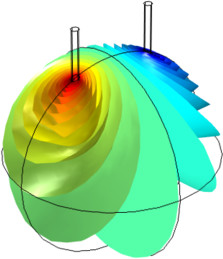
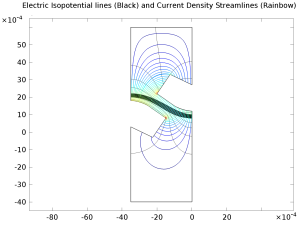
The electrochemical cell shown in this model can be regarded as a unit cell of a larger wire-mesh electrode that is common in many industrial processes. One of the most important aspects in the design of electrochemical cells is the current density distributions in the electrolyte and ... En savoir plus
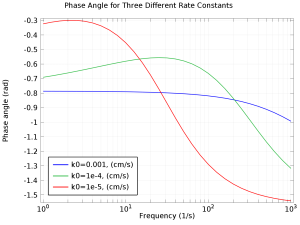
The purpose of this app is to understand EIS, Nyquist, and Bode plots. The app lets you vary the bulk concentration, diffusion coefficient, exchange current density, double layer capacitance, and the maximum and minimum frequency. Electrochemical impedance spectroscopy (EIS) is a common ... En savoir plus
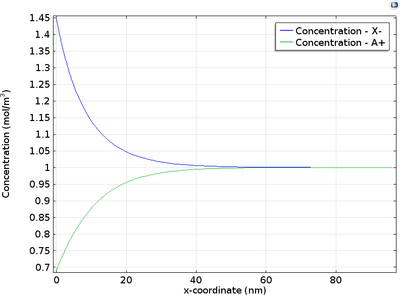
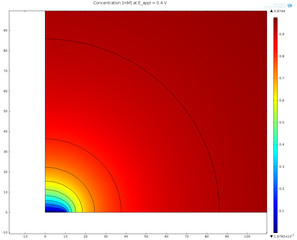
At the electrode-electrolyte interface, there is a thin layer of space charge in a diffuse double layer. This may be of interest when modeling devices such as electrochemical capacitors and nanoelectrodes. This tutorial example shows how to couple the Nernst-Planck equations to the ... En savoir plus
This tutorial example models the currents and the concentration of dissolved metal ions in a battery (corrosion cell) made from an orange and two metal nails. This type of battery is commonly used in chemistry lessons. Instead of an orange, lemons or potatoes can also be used. En savoir plus
The chlor-alkali membrane process is one of the largest in industrial electrolysis with the production of roughly 40 million metric tons of both chlorine and caustic soda per year. Chlorine is used predominantly for the production of vinyl chloride monomer, which in turn is used for the ... En savoir plus
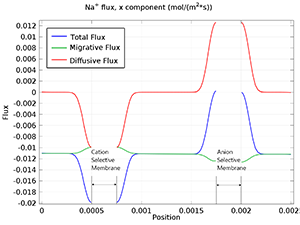
Electrodialysis is a separation process for electrolytes based on the use of electric fields and ion selective membranes. Some common applications of the electrodialysis process are: Desalination of process streams, effluents, and drinking water pH regulation in order to remove acids ... En savoir plus
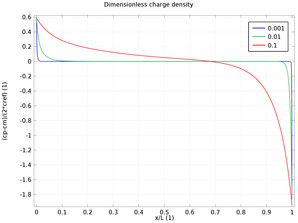
In the diffuse double layer and within the first few nanometers of an electrode surface, the assumption of electroneutrality is not valid due to charge separation. Typically, the diffuse double layer may be of interest when modeling very thin layers of electrolyte including those in ... En savoir plus
Voltammetry is modeled at a microelectrode of 10um radius. In this common analytical electrochemistry technique, the potential at a working electrode is swept up and down and the current is recorded. The current-voltage waveform ("voltammogram") gives information about the reactivity and ... En savoir plus
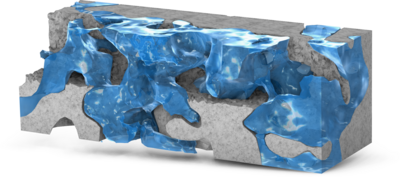
Digital Rock technology utilizes high-resolution imaging techniques, such as SEM and X-ray CT, to characterize the pore structures of rock cores. This approach enables pore-scale modeling of reservoir rocks, offering valuable insights into pore morphology, connectivity, and fluid ... En savoir plus