La Bibliothèque d'Applications présente des modèles construits avec COMSOL Multiphysics pour la simulation d'une grande variété d'applications, dans les domaines de l'électromagnétisme, de la mécanique des solides, de la mécanique des fluides et de la chimie. Vous pouvez télécharger ces modèles résolus avec leur documentation détaillée, comprenant les instructions de construction pas-à-pas, et vous en servir comme point de départ de votre travail de simulation. Utilisez l'outil de recherche rapide pour trouver les modèles et applications correspondant à votre domaine d'intérêt. Notez que de nombreux exemples présentés ici sont également accessibles via la Bibliothèques d'Applications intégrée au logiciel COMSOL Multiphysics® et disponible à partir du menu Fichier.
This 2D model demonstrates how to model a galvanic couple in which the corrosion of the anode causes a geometry deformation. The parameter data used is for an Magnesium Alloy (AE44) - mild steel couple in brine solution (salt water). En savoir plus
This model demonstrates the "butterfly" filling mechanism for copper electrodeposition in a Through-Hole (TH) via exposed to an electrolyte containing halide-suppressor additives. The Tertiary Current Distribution, Nernst Planck interface in combination with Deformed Geometry is used ... En savoir plus
This tutorial explores how pulse reverse plating can be used as an additive-free alternative to attenuate small protrusions during copper metal deposition. By matching the process parameters, including the length of the forward and reverse pulses (duty cycles), a bright mirror-like metal ... En savoir plus
This app demonstrates the usage of a surrogate model function for predicting the cell voltage, cell open circuit voltage and internal resistance of an NMC111/graphite battery cell undergoing a battery test cycle. The surrogate function, a Deep Neural Network, has been fitted to a ... En savoir plus
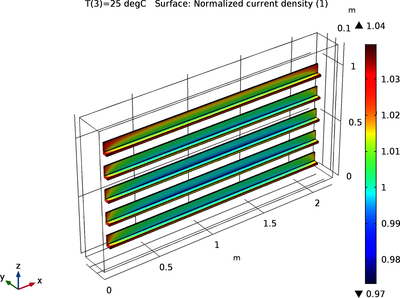
In a redox flow battery electrochemical energy is stored as redox couples in the electrolyte, which is stored in tanks outside the electrochemical cell. During operation, electrolyte is pumped through the cell and, due to the electrochemical reactions, the individual concentrations of ... En savoir plus
When anodizing aluminum, the surface is electrochemically altered to form an abrasive and corrosion-resistive Al2O3 film. The electrode kinetics during the process are only marginally affected as the oxide layer grows, so a stationary analysis of the current distribution is sufficient to ... En savoir plus
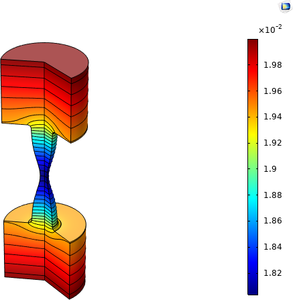
Oxide jacking is the process by which reinforced concrete cracks, due to the corrosion of the reinforcing rebar rods. The corrosion process causes growth of an oxide layer on the rebar, which in turn causes internal stresses in the concrete. If the corrosion process is allowed to ... En savoir plus
The present model example is based on Copper Deposition in a Trench model available in Electrodeposition Application Library. The nonuniform deposition along the trench surface leads to formation of a cavity/void. Since the Deformed Geometry interface cannot handle topological changes, ... En savoir plus
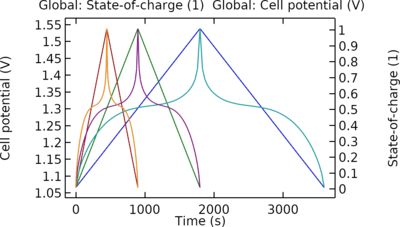
Zinc-Silver oxide (Zn-AgO) batteries are used in different industries due to their high capacity per unit weight. In this work, discharge of a Zn-AgO battery is simulated using the Battery with Binary Electrolyte interface. The electrochemical reactions in the positive and negative ... En savoir plus
A simple equivalent circuit model approach is presented for Nickel metal hydride batteries. The 0D model consists of resistor, capacitor, current source and state-of-charge based voltage source (SOC). An Arrhenius type dependence is used to account for self-discharge. All model ... En savoir plus